

iTind Procedure
Managing lower urinary tract symptoms in patients with BPH is a delicate juggling act. Symptom relief is of paramount importance, but as more aging patients maintain very active lifestyles, preserving this and sexual function are of growing importance to them. With the iTind procedure, patients do not have to compromise. iTind is a new alternative to medications and their side effects, and is a minimally-invasive alternative to surgery.
The iTind treatment is a straightforward procedure that provides rapid symptom relief in the majority of patients. The procedure can be performed in an office or as an outpatient hospital procedure, under general or local anesthesia. Patients typically return home the same day and do not require a catheter. For patients who prefer a non-pharmaceutical approach to treating their BPH symptoms, or who do not want or cannot undergo surgery, iTind is well suited to become a first line treatment.
ITIND IS AN IDEAL SOLUTION FOR PATIENTS WHO:
- Are not happy with medical therapy
- Do not want surgery
- Want to preserve sexual function
- Want a rapid return to daily life
ITIND IS SUITABLE FOR PATIENTS WITH:
- Prostate volume <75ml
- No obstructive median lobe
- High bladder neck
- Good bladder function
BENEFITS
- Bloodless incisions create permanent remodeling of prostatic urethra
- Preserves sexual function
- Temporary implant, removed after only 5 days
- Rapid symptoms relief, rapid return to daily life
- Straightforward procedure with no need for special equipment
- No post-op catheterization
DEVICE COMPONENTS
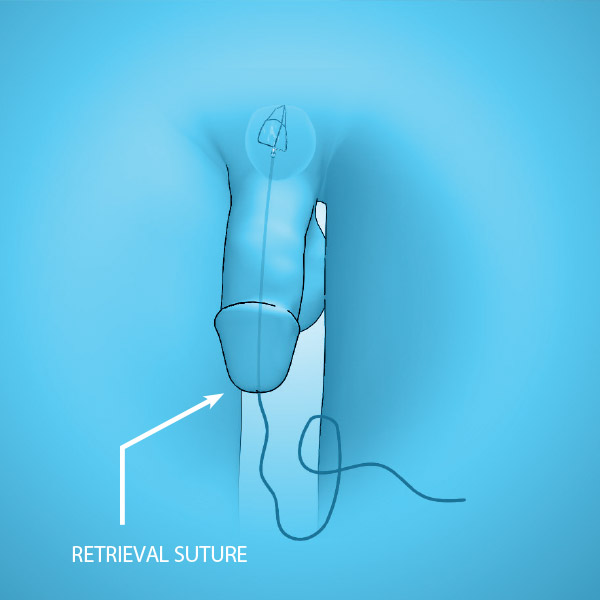
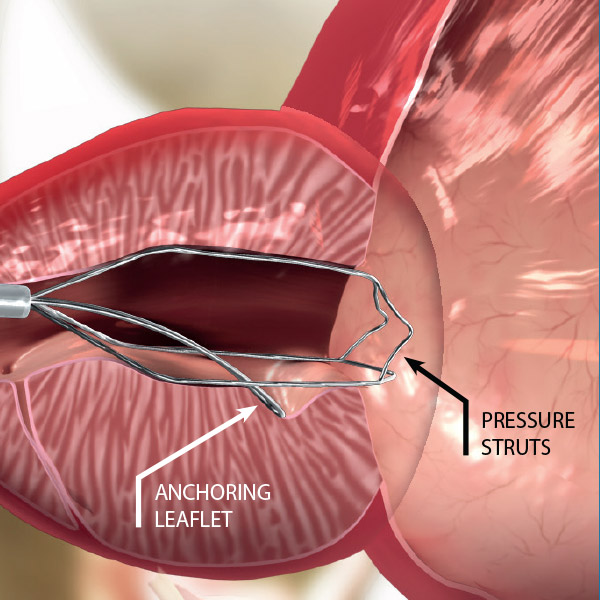
- Three nitinol pressure struts at 12, 5 and 7 o’clock
- Anti-migration anchoring leaflet
- Retrieval suture for easy removal
Mechanism of Action
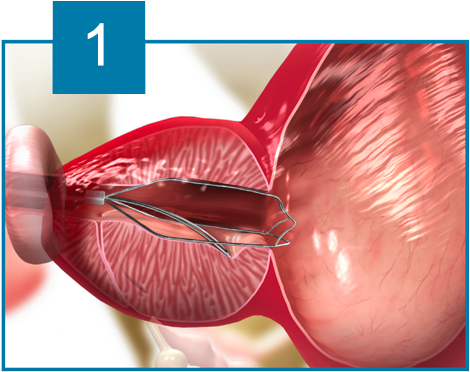
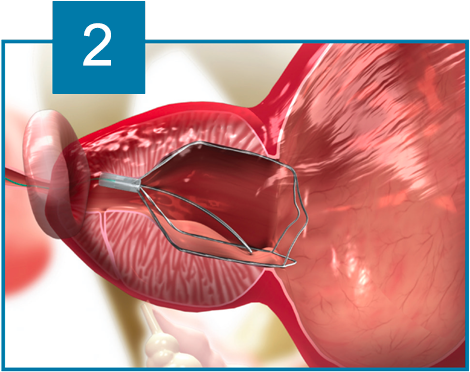
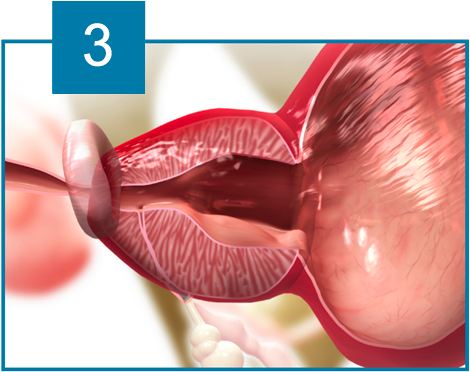
The iTind device is placed within the prostatic urethra in a folded configuration. Once in place, the iTind expands and its pressure struts exert longitudinal force on the prostatic urethra and the bladder neck at the 12, 5 and 7 o’clock positions.
Over the course of 5 to 7 days, ischemia, necrosis and scarring, create deep longitudinal incisions through which urine can flow freely.
The device design ensures easy and accurate positioning. The ischemic incisions preserve the integrity of the muscles of the bladder neck, prostatic urethra and external urethral sphincter, thereby eliminating the risk of retrograde ejaculation, erectile dysfunction and urinary incontinence.
At post-operative day 5 to 7, the iTind is completely removed. The new incisions formed continue to provide relief over the long-term as demonstrated in clinical testing out to 3 years.
Clinical Data
The iTind was developed as a means of minimally invasively treating the symptoms of BPH, without the risks associated with surgery, without burning tissue, and without leaving behind a permanent implant.
Clinical data from two peer-reviewed studies have demonstrated that the iTind procedure is effective, well tolerated and preserves sexual function. Rapid and significant improvement in lower urinary tract symptoms, flow and quality of life have been observed, and durable improvement for 3 years.
PUBLICATIONS
Porpiglia, et al. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at one year of follow-up. British Journal of Urology International. 2015.
View Abstract >>
Porpiglia, et al. Three year follow up temporary implantable nitinol device (TIND) implantation for the treatment of benign prostatic obstruction.
British Journal of Urology International. 2018.
View Abstract >>
Porpiglia et al. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multi-centre study at 1 year of follow-up. British Journal of Urology International. 2018.
View Abstract >>
Kadner et al. Second generation of temporary implantable nitinol device (iTind) in men with LUTS: 2 year results of the MT-02-study. World Journal of Urology. 2020.
View Abstract >>


